Out of sight, but not out of mind: Environmental Scientists discuss PFAS in the world around us
UArizona experts explain what the concern around ‘Forever Chemicals’ is, how they move through the environment, and what we can do about them
PFAS Pan

Often associated with household items like non-stick pans, PFAS are garnering attention once again as the Environmental Protection Agency (EPA) recently reduced health advisory limits by 99.99% in drinking water. Research on PFAS continues to unveil new findings at the University of Arizona, and some of the scientists behind this work answered common questions about the chemicals.
Meet the Scientists
Jon ChoroverEnvironmental Science Department Head, Director of Arizona Lab for Emerging Contaminants (ALEC) |
Mark BrusseauProfessor, Department of Environmental Science |
Ian PepperRegents Professor, Department of Environmental Science, Director of the Water & Energy Sustainable Technology (WEST) Center |
|---|---|---|
|
Chorover’s research focuses on analyzing the chemical makeup and reactions of soil and water contaminants, as well as transport and fate. He is a University of Arizona faculty member of more than 20 years and received his PhD in Soil and Water Chemistry from UC Berkeley. |
Brusseau’s research focuses on soil and groundwater contamination and remediation. Part of that includes how contaminants move through the environment. He is an Environmental Science faculty member with more than a decade of PFAS research, and earned his PhD in Subsurface Hydrology and Environmental Chemistry from the University of Florida. |
Pepper’s research focuses on soil and water quality related to human health, with a specialty in wastewater contaminants. He published nearly 180 peer-reviewed journal articles, over 100 book chapters and nine textbooks. He received his PhD in Soil Microbiology from Ohio State University. |
The PFAS Issue
“We have this joke in the science community,” Pepper started the interview, “If you don’t find PFAS in your samples, you are measuring it wrong.”
Despite the lab humor, there is truth to the statement as well. Nearly all humans in the U.S. are assumed to have some PFAS in their blood, as it accumulates over time from varying sources including drinking water.
On June 15th, 2022, the EPA announced new health advisory limits for the concentration of two PFAS molecules — PFOA and PFOS — in drinking water. The new advisory, according to Pepper, is confusing.
“Prior to 2016 the advisory level of PFAS was 600 parts-per-trillion (ppt) then in 2016 it was 70ppt, and now 0.004ppt, which is insane,” Pepper said. “Even with our fancy equipment we can’t even measure at that level, so basically any detection of PFAS in water will be above this advisory.”
Some studies show PFAS have negative health effects to humans, though Pepper said there is still a lot we do not understand.
“Our knowledge of the health effects has been a progression,” Pepper said. “At first, it was ‘Maybe it causes health effects.’ Now they suspect one of them to be a carcinogenic agent in the thyroid glands. But more long-term, low-dose studies on humans are needed.”
Although the concentration of PFOS and PFOA in human blood have decreased significantly over the past two decades, the U.S. average in 2018 was estimated at about 4300ppt and 1400ppt, respectively.
A part-per-trillion measurement can be difficult to grasp due to how much one trillion of anything is. One ppt of a billionaire’s wealth would be less than a penny. More closely related to PFAS, one ppt equates to about one drop of water in 20 Olympic-sized swimming pools.
Defining PFAS
PFAS (pronounced PEA-fass) is an acronym that stands for per- and poly-fluoroalkyl substances. More simply put, Chorover describes them as, “a remarkably large suite of molecules that are anthropogenic, meaning they are human-created.”
PFAS were first introduced and became commonly used in the 1950s in household products like non-stick pans, stain-resistant carpets, and water-resistant clothing, amongst others.
These chemicals are surfactants, meaning they reduce the surface tension between substances. Another common use of surfactants is in household detergents, where the compounds are used to stir up activity on the surface to help trap dirt and remove it from surfaces.
Although the surfactant quality of PFAS is beneficial in some circumstances, they also can pose health risks to humans as the molecules do not easily degrade in the environment, earning their nickname, “Forever Chemicals”.
“The surfactant properties are why PFAS were produced in such large numbers,” Chorover said. “But now we also recognize, unfortunately, which is often the case in human endeavors, that there is an environmental and a human-health downside. These compounds are now being recognized as toxic to human health, though the precise mechanisms of toxicity are not fully understood yet.”
Tracking PFAS in the Environment
Brusseau’s research focuses on transport and fate of contaminants such as PFAS, in scales ranging from microscopic to entire contaminated sites. By scanning soil samples at the microscopic scale, much like humans use CAT scans, Brusseau’s lab can understand how contaminants migrate and end up in humans.
PFAS Movement Diagram
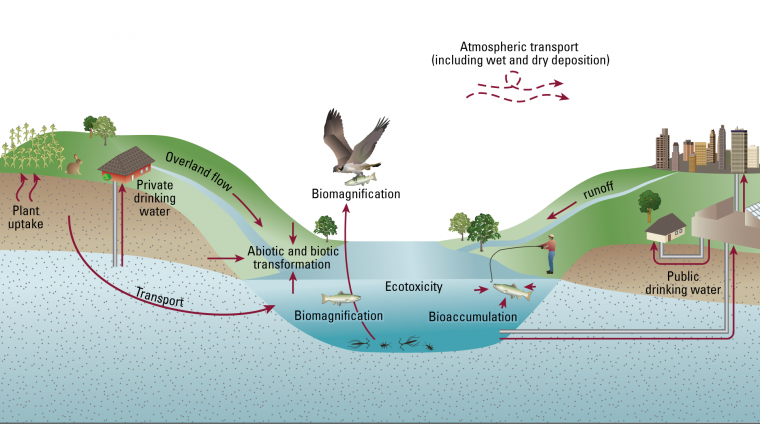
“PFAS are found in soil across the globe and that means there is high potential for exposure for humans and other organisms,” Brusseau said. “One possible route is leaching to groundwater, and if people are using groundwater as potable water supply then they could be exposed. Another possible route is if plants are grown in contaminated soil, and those plants are used to feed animals or for human consumption.”
After humans consume PFAS molecules, some stay in the body while the rest leave through biowaste. Pepper’s research tracks contaminants in wastewater. The issue, Pepper said, is their surfactant quality causes PFAS to move from wastewater to biosolids, which are then spread across U.S. land.
PFAS in biosolids paired with the new EPA health advisory has caused new scrutiny of land application of biosolids, Pepper said.
“60 percent of biosolids are land-applied,” Pepper said. “So, what are the alternatives? Landfill or incinerate. That’s it. Neither of those are good.”
Pepper said landfills will lead to higher rates of leaching to groundwater while wasting organic carbon, and incineration typically leads to increased air pollution.
Fixing the PFAS Issue
Consuming PFAS through drinking water can be reduced by using filters that use activated carbon. On a larger scale, this is how many treatment plants also clean water, according to Chorover.
“The traditional remediation scheme is pump and treat, where you pump the wells to bring the water up with the contaminant in it and you treat the contamination on the surface basically on a big bed of activated carbon,” Chorover said. “Then you pump the water back down into the aquifer. It's a very energy intensive and expensive process.”
As health advisory limits lower, this filtration process becomes more intensive, leading to higher energy usage and costs. Chorover said his lab is working on coming up with a more efficient solution.
“We are working on synthesizing very small polymers that have a high affinity for PFAS and can kind of suck the PFAS out of the water by adsorption,” Chorover said. “We want to inject these benign polymers, a green technology that doesn’t have any known negative environmental consequences, into sand and gravel aquifers and lure the PFAS out of the water.”
To help narrow down where remediation efforts are needed most, Brusseau is working on creating models that predict how PFAS will move and the likelihood of leaching to occur.
“We are developing mathematical models that are designed specifically for these compounds because they have some properties that make their transport and fate behavior different from the more traditional contaminants we have been working with for decades,” Brusseau said.
Brusseau and a colleague already published one simplified model that is being used at contaminated sites, but is continuing to strengthen the models through field and laboratory research. He plans on taking a Tucson soil monolith, about a meter cubed and weighing multiple tons, to the WEST Center to simulate PFAS spills.
“That is the process — we conduct laboratory research, then we collect data from actual contaminated sites and use all this information to implement the model,” Brusseau said. “Then we see if our model accurately represents what is observed at field sites. Our lab has been working like this on PFAS for over 10 years.”
PFAS in Tucson
The Environmental Scientists at the University of Arizona often use Tucson soil and water samples in their research. Pepper has been testing Tucson biosolids and land application for 20 years, with funding from Pima County. Pepper, Chorover, and Brusseau all recognize a few Tucson locations with high concentrations of PFAS.
AFFF

“It's very prevalent in certain areas of Tucson groundwater, for example near the Davis-Monthan Airforce Base,” Chorover said. “Military bases tend to be locations with significant amounts of PFAS in the soil from their fire extinguishing practices.”
A major component of military fire extinguishing methods includes using Aqueous Film Forming Foam (AFFF), which contains PFAS. Other highly contaminated sites, according to Brusseau, are in Marana and near the Tucson International Airport. The Town of Marana Water Department released a statement regarding PFAS in town water, and the Arizona Department of Environmental Quality shares resources on the PFAS situation in Tucson and Arizona as a whole.
Final Remarks on PFAS
Other than using activated carbon water filters, there are other ways to avoid accidentally consuming PFAS. According to Pepper, one of these is watching out for a common occurrence at home.
“If you look at routes of exposure, one of the greatest routes of exposure is household dust,” Pepper said. “If you are worried, what you really need to do is get rid of all the carpets in your home. Household dust is loaded with the stuff.”
Although there is a significant amount of research on PFAS, there are still many unknowns for environmental scientists to discover at the University of Arizona. Chorover said this includes figuring out how much PFAS humans are truly exposed to.
PFAS molecules contain fluorine, so by measuring random samples of water along with known quantities of PFAS, the total amount of fluorine should just be from the known PFAS. However, Chorover is finding there is much more fluorine than just the known PFAS.
“It is important to get a handle on that total organic fluorine because that is a more accurate representation of the total exposure that we are having to PFAS and PFAS related compounds,” Chorover said.
With Chorover, Pepper, and Brusseau studying unique aspects of PFAS, along with several other Environmental Science faculty, we are answering many of the questions PFAS poses. Over the past couple decades, our knowledge of PFAS, their health effects, and their behavior grew immensely and will continue to grow in the future.



